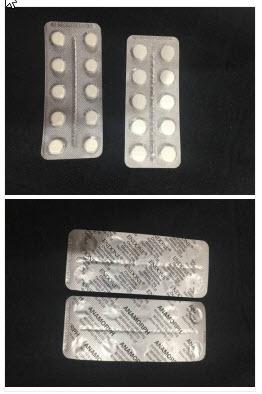
A recall notice for Endone Oxycodone Hydrochloride 5mg Tablet Blister Packs supplied by Aspen Pharma after a much stronger drug was reported to have been found in a packet.
Aspen Pharma Pty Ltd — Endone Oxycodone Hydrochloride 5mg Tablet Blister Pack
PRA No.
2019/17727
Date published
31 Jul 2019
Product description
Endone oxycodone hydrochloride 5mg tablet blister pack
Batch Number: CW612
Expiry: Nov 2020
AUST R: 14945
Identifying features
Other
Batch Number CW612
What are the defects?
A report has been received that a strip of 30mg Anamorph tablets may have been present in a box of 5mg Endone, post dispensing.
What are the hazards?
Anamorph tablets are approximately four times the Endone equivalent dose, so there is the potential of serious health risks and overdose, if taken inadvertently.
What should consumers do?
Consumers should visually inspect blister sheets to verify both blister strips contain Endone tablets. If a blister is identified with Anamorph printed on the back, do not use the tablets from that strip. Return strips of incorrect tablets to the chemist or pharmacy from which they were purchased.
Further information is available on the TGA website at: https://www.tga.gov.au/alert/endone-5-mg-tablets
For further information, contact Aspen Pharma by phone on 1300 659 646 or email at medical@aspenpharmacare.com.au
Supplier
Traders who sold this product
Pharmacies and Chemists
Where the product was sold
Nationally
Responsible regulator
Therapeutic Goods Administration is the responsible regulator for this recall.
Wow. Now even the “Hillbilly Herion” cannot be trusted as being safe.